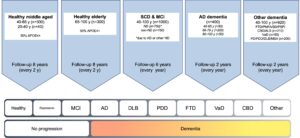
Overview
Cognitively healthy middle-aged individuals (Cohort A)
ENROLLMENT
A novelty in BioFINDER2 is that we include a relatively young cohort of cognitively healthy people. The purpose of this is to be able to detect the earliest stages of pathology in neurodegenerative diseases, especially Alzheimer’s disease, which is believed to start several decades before dementia. We recruit 300 cognitively healthy individuals, where we aim to include as many individuals as possible that participate in the Malmö Offspring study, which is an epidemiological study consisting of the children and grandchildren of the study participants in the Malmö Diet and Cancer study. The participants are enriched for APOE e4 carriers, with approximately 50% carrying at least one APOE e4 allele. The enrichment of people positive for the APOE e4 allele will increase our chances to detect brain changes in the earliest stages of Alzheimer’s disease. At the same time, the relatively young age of this cohort will minimize the influence of age-related changes which may otherwise confound the relationship between cognition and brain changes associated with AD.
INCLUSION CRITERIA
- Age 40-65 years
- Absence of cognitive symptoms as assessed by a physician with special interest in cognitive disorders.
- MMSE score 27-30 at screening visit.
- Do not fulfill the criteria for MCI or any dementia according to DSM-V.
- Speaks and understands Swedish to the extent that an interpreter is not necessary for the patient to fully understand the study information and cognitive tests.
EXCLUSION CRITERIA
- Significant unstable systemic illness or organ failure, such as terminal cancer, that makes it difficult to participate in the study.
- Current significant alcohol or substance misuse.
- Significant neurological or psychiatric illness.
- Refusing lumbar puncture, MRI or PET.
FOLLOW-UP FOR 8 YEARS
Every 2 years new clinical, cognitive, neurological, and psychiatric assessments as well as CSF/blood sampling, Tau PET, Amyloid PET, and MRI are performed in all cases as described in table below (except for PET in a subgroup of amyloid negative participants).
Participants with suspicion of parkinsonian disorder during follow-up perform additional examinations (e.g., FE-PE2I-PET and skin biopsy) related to α-synucleinopathy in the same manner as cohort E parkinsonian disorder do. Suspicion is determined as biomarker-verified α-synucleinopathy.
AUXILIARY COHORT (Cohort A2)
We recruit 40 healthy individuals aged 20-40 years (20 with APOE e4 genotype and 20 without APOE e4) from the Malmö Offspring study using the same inclusion criteria (besides age) and exclusion criteria, as well as follow-up, as written above. We need to include some younger cases to be able to determine cut offs for normal values for PET and CSF biomarkers, because some AD pathology (especially tau aggregates in the medial temporal lobe) can occur already at the age of 40-50 years in asymptomatic individuals. However, the follow-up in this younger cohort is slightly different compared to cohort A. To reduce the total exposure of radiation in this younger population (20-40 years) we only perform new Tau PET and Amyloid PET at most at two occasions during follow-up.
Cognitively healthy elderly individuals (Cohort B)
ENROLLMENT
We recruit 300 cognitively healthy individuals, where we aim to include as many individuals as possible that did participate in the Malmö Diet and Cancer study during the early 1990’s. The participants are enriched for APOE e4 carriers, with approximately 50% carrying at least one APOE e4 allele.
INCLUSION CRITERIA
- Age 66-100 years
- Absence of cognitive symptoms as assessed by a physician with special interest in cognitive disorders.
- MMSE score 26-30 at screening visit.
- Do not fulfill the criteria for MCI or any dementia according to DSM-V.
- Speaks and understands Swedish to the extent that an interpreter is not necessary for the patient to fully understand the study information and cognitive tests.
EXCLUSION CRITERIA
- Significant unstable systemic illness or organ failure, such as terminal cancer, that makes it difficult to participate in the study.
- Current significant alcohol or substance misuse.
- Significant neurological or psychiatric illness.
- Refusing lumbar puncture, MRI or PET.
FOLLOW-UP FOR 8 YEARS
Every 2 years new clinical, cognitive, neurological, and psychiatric assessments as well as CSF/blood sampling, Tau PET, Amyloid PET, and MRI are performed in all cases as described in table below (except for PET in a subgroup of amyloid negative participants).
Participants with suspicion of parkinsonian disorder during follow-up perform additional examinations (e.g., FE-PE2I PET and skin biopsy) related to α-synucleinopathy in the same manner as cohort E parkinsonian disorder do. Suspicion is determined as biomarker-verified α-synucleinopathy.
Subjective cognitive decline and mild cognitive impairment (Cohort C)
ENROLLMENT
Up to 800 patients with either subjective cognitive decline (SCD) or mild cognitive impairment (MCI) are recruited in a consecutive fashion from the Skåne University Hospital and Ängelholm Hospital in southern Sweden. We predominantly include cases where the medical doctor believes that the cognitive symptoms are caused by an incipient neurocognitive disorder. For example, all cases with evidence of brain amyloid pathology (i.e., an abnormal CSF Aβ42/40 ratio) are included.
INCLUSION CRITERIA
- Age 40-100 years.
- Referred to the memory clinics due to cognitive symptoms experienced by the patient and/or informant. These symptoms do not have to be memory complaints, but could also be executive, visuospatial, language, praxis, psychomotor or social cognitive complaints.
- MMSE score of 24 – 30 points.
- Do not fulfill the criteria for any dementia (major neurocognitive disorder) according to DSM-5.
- The medical doctor (after clinical assessments, cognitive testing, CSF analyses and structural brain imaging) believes the cognitive complaints are caused by an incipient neurocognitive disorder of any sort. This is defined as any case fulfilling the criteria above (i.e. both SCD and MCI) with an abnormal CSF Aβ42/40 ratio, which is strongly associated with brain Aβ pathology and prodromal Alzheimer’s disease. Further, cases with MCI (=minor neurocognitive impairment) due to either Parkinson’s disease, Lewy body disease, vascular neurocognitive disorder or frontotemporal dementia can also be included.
- Speaks and understands Swedish to the extent that an interpreter is not necessary for the patient to fully understand the study information and cognitive tests.
EXCLUSION CRITERIA
- Significant unstable systemic illness or organ failure, such as terminal cancer, that makes it difficult to participate in the study.
- Current significant alcohol or substance misuse.
- Refusing lumbar puncture, MRI or PET.
FOLLOW-UP FOR 6 YEARS
Every 12 months new clinical, cognitive, neurological, and psychiatric assessments will be performed. CSF/blood sampling, Tau PET, Amyloid PET, and MRI are performed every 2 years in all cases as described in table below (except for PET in a subgroup of amyloid negative participants).
Participants with suspicion of parkinsonian disorder during follow-up perform additional examinations (e.g., FE-PE2I-PET and skin biopsy) related to α-synucleinopathy in the same manner as cohort E parkinsonian disorder do. Suspicion is determined as biomarker-verified α-synucleinopathy.
AUXILIARY COHORT (Cohort C2)
Up to 250 additional cases with SCD/MCI where the medical doctor does not suspect incipient neurocognitive disorder, undergo the same baseline investigations as the rest of Cohort C. However, they perform clinical follow up only every second year to definitely rule out ongoing neurocognitive disorder. CSF/blood sampling, Tau PET, Amyloid PET, and MRI are performed every 2 years in all cases as described in table below (except for PET in a subgroup of amyloid negative participants). For these subjects to be eligible the medical doctor (after clinical assessments, cognitive testing, CSF analyses and structural brain imaging) believes the cognitive complaints are not caused by an incipient neurocognitive disorder of any sort. The following criteria needs to be fulfilled for SCD/MCI cases to be included in cohort C2: i) the subject has a normal CSF Aβ42/40 ratio, ii) a normal DaTSCAN and/or the symptomatology is not in agreement with incipient Parkinson’s disease dementia (PDD) or Dementia with Lewy Bodies (DLB)), iii) symptoms and imaging-findings suggesting neither incipient Frontotemporal Dementia (FTD) nor incipient Vascular Dementia (VaD). In similarity to Cohort C participants with biomarker-verified suspicion parkinsonian disorder during follow-up undergo additional examinations (see above).
Dementia due to Alzheimer’s disease (Cohort D)
ENROLLMENT
We recruit 400 patients with mild to moderate dementia due to Alzheimer’s disease (AD) from the Skåne University Hospital and Ängelholm Hospital in southern Sweden. At least but not restricted to 50 cases aged 40-65 years of age, 200 cases aged 66-79 years of age and 50 cases aged 80-100 years of age are included, with a maximum of 400 cases in total.
INCLUSION CRITERIA
- Age 40-100 years.
- Referred to the memory clinics due to cognitive symptoms experienced by the patient and/or informant. These symptoms do not have to be memory complaints, but could also be executive, visuospatial, language, praxis or psychomotor complaints.
- MMSE score of 12-30 points.
- Fulfill the criteria for dementia (major neurocognitive disorder) due to Alzheimer’s disease (DSM-V).
- Speaks and understands Swedish to the extent that an interpreter was not necessary for the patient to fully understand the study information and cognitive tests.
EXCLUSION CRITERIA
- Significant unstable systemic illness or organ failure, such as terminal cancer, that makes it difficult to participate in the study.
- Current significant alcohol or substance misuse.
- Refusing lumbar puncture, MRI or PET.
FOLLOW-UP FOR 2 YEARS
Every 12 months new clinical, cognitive, neurological, and psychiatric assessments are performed. CSF/blood sampling, Tau PET, and MRI are performed at baseline and after 2 years as described in table below. No Amyloid PET is performed in this group.
Participants with suspicion of parkinsonian disorder during follow-up perform additional examinations (e.g., FE-PE2I PET and skin biopsy) related to α-synucleinopathy in the same manner as cohort E parkinsonian disorder do. Suspicion is determined as biomarker-verified α-synucleinopathy.
Other dementias (Cohort E)
ENROLLMENT
Patients with primary neurodegenerative disorders other than Alzheimer’s disease will be recruited from the Memory Clinic and Neurology clinic at Skåne university hospital and Ängelholm hospital in southern Sweden. We will include 920 cases including following diagnoses:
- Frontotemporal dementia (FTD)-related disorders, including behavioral variant of FTD (bvFTD) (n >50), Progressive nonfluent aphasia (PNFA) (n >20), semantic dementia (SD) (n >20), Progressive supranuclear palsy (PSP) (n >50), Corticobasal degeneration (CBD) (n >20), and Amyotrophic lateral sclerosis (ALS) (n >50).
- Subcortical Vascular dementia (VaD) (n >50).
- Parkinson’s disease (PD) (n >70), Parkinson’s disease with dementia (PDD) (n >30), Dementia with Lewy Bodies (DLB) (n >70), Multiple system atrophy (MSA) (n >30).
INCLUSION CRITERIA
- Age 40-100 years.
- Fulfill the criteria for dementia (major neurocognitive disorder) due to FTD, PDD, DLB or subcortical VaD alternatively the criteria for PD, PSP, MSA, CBS or ALS.
- Speaks and understands Swedish to the extent that an interpreter was not necessary for the patient to fully understand the study information and cognitive tests.
EXCLUSION CRITERIA
- Significant unstable systemic illness or organ failure, such as terminal cancer, that makes it difficult to participate in the study.
- Current significant alcohol or substance misuse.
- Refusing lumbar puncture, MRI or PET.
FOLLOW-UP FOR 2 YEARS
Depending on diagnosis, i.e., parkinsonian disorder or not, at inclusion different examination protocols for both baseline and follow up are used as described in tables below. Every 12 months new clinical, cognitive, neurological, and psychiatric assessments will be performed. For the ALS subcohort it will be performed twice a year. CSF/blood sampling, Tau PET (depends on further funding) and MRI will be done at baseline and after 2 years. No Amyloid PET in this group. The ALS subcohort will perform blood sampling twice a year whereas CSF sampling, Tau PET, MRI, and neurophysiologic examination will be performed every year.
Participants (in group 1 and 2 above) with suspicion of parkinsonian disorder during follow-up perform additional examinations (e.g., FE-PE2I-PET and skin biopsy) related to α-synucleinopathy in the same manner as cohort E parkinsonian disorder do. Suspicion is determined as biomarker-verified α-synucleinopathy.
Table Cohort A & B
Follow-up of healthy individuals (Cohorts A and B)
| Baseline | 2 y | 4 y | 6 y | 8 y | |
| MD/Nurse | MD/Nurse | MD/Nurse | MD/Nurse | ||
| MRI | x | x | x | x | x |
| Tau PET† | x | x | x | x | x |
| Amyloid PET† | x | x | x* | x | x* |
| Lumbar puncture | x | x | x | x | x |
| Plasma/Blood | x | x | x | x | x |
| Skin biopsy / Cell brush swab | x | x | x | ||
| Basic Demographics | x | ||||
| BAS+ and Sleep questionnaires | x | x | x | x | |
| ADL (FAQ) | x | x | x | x | x |
| CIMP-QUEST | x | x | x | x | x |
| MBI (apathy) | x | x | x | x | x |
| EQ-5D | x | x | x | x | x |
| SCOPA-AUT | x | x | |||
| RBD screening questionnaire (RBDSQ) | x | x | |||
| SCOPA-sleep | x | x | |||
| NMSQuest | x | x | |||
| MMSE | x | x | x | x | x |
| AQT | x | x | x | x | x |
| TMTA | x | x | x | x | x |
| TMTB (if MMSE ≥20) | x | x | x | x | x |
| ADAS 10 word recall + recognition + BNT15 | x | x | x | x | x |
| SDMT | x | x | x | x | x |
| Letter S fluency | x | x | x | x | x |
| Animal fluency | x | x | x | x | x |
| VOSP incomplete letters | x | x | x | x | x |
| VOSP cube analysis | x | x | x | x | x |
| FCSRT | x | x | x | x | x |
| Computerized cognitive battery | x | x | x | x | x |
| Sniffin’ Sticks test | x | x | |||
| Global Deteriorating Scale | x | x | x | x | x |
| HADS | x | x | x | x | x |
| Motor aspects and visuo-spatial navigation (Cohort B) | x | x | x |
* Do not include participants between ages 20-40 years. † Reduced scheme for a subgroup of amyloid negative participants (see PET table below).
SCOPA-AUT, RBDSQ, SCOPA-SLEEP, NMSQuest, and Sniffin’ Sticks test were incorporated from 2019-JUN-10
Table Cohort C
Follow-up of SCD & MCI with suspected ND (Cohort C)
| First visit | Diagnosis-Baseline | 1 y | 2 y | 3 y | 4 y | 5 y | 6 y | |
| MD/Nurse | MD | MD/Nurse | MD/Nurse | Nurse | MD/Nurse | Nurse | MD/Nurse | |
| MRI | x | x | x | x | ||||
| Tau PET† | x | x | x | x | ||||
| Amyloid PET† | x | x | x | x | ||||
| Lumbar puncture | x | x | x | x | ||||
| Plasma/Blood | x | x | x | x | ||||
| Basic Demographics | x | |||||||
| BAS+ and Sleep questionnaires | x | x | x | x | x | x | ||
| CIMP-QUEST | x | x | x | x | x | x | x | |
| ADL | x | x | x | x | x | x | x | |
| EQ-5D (VAS also informant) | x | x | x | x | ||||
| MBI-C | x | p.r.n. | x | p.r.n. | x | p.r.n. | x | |
| MMSE | x | x | x | x | x | x | x | |
| AQT | x | x | x | x | x | x | x | |
| TMTA | x | x | x | x | x | x | x | |
| TMTB (if MMSE ≥20) | x | x | x | x | x | x | x | |
| ADAS 10 word recall + recognition + BNT15 | x | x | x | x | x | x | x | |
| SDMT | x | x | x | x | x | x | x | |
| Letter S fluency | x | x | x | x | x | x | x | |
| Animal fluency | x | x | x | x | x | x | x | |
| VOSP incomplete letters | x | x | x | x | x | x | x | |
| VOSP cube analysis | x | x | x | x | x | x | x | |
| HADS | x | x | x | x | x | x | x | |
| Motor aspects and visuospatial navigation | x | x | x |
† Reduced scheme for a subgroup of amyloid negative participants (see PET table below).
p.r.n.: pro re nata, when needed.
Table Cohort C2
Follow-up of SCD & MCI without suspected ND (Cohort C2)
| First visit | Diagnosis baseline | 2 y | 4 y | 6 y | |
| MD/ Nurse | MD | MD/ Nurse | MD/ Nurse | MD/ Nurse | |
| MRI | x | x | x | x | |
| Tau PET† | x | x | x | x | |
| Amyloid PET† | x | x | x | x | |
| Lumbar puncture | x | x | x | x | |
| Plasma/Blood | x | x | x | x | |
| Basic Demographics | x | ||||
| BAS+ and Sleep questionnaires | x | x | x | x | |
| CIMP-QUEST | x | x | x | x | |
| ADL | x | x | x | x | |
|
EQ-5D (VAS also informant) |
x | x | x | x | |
| MBI-C | x | x | x | x | |
| MMSE | x | x | x | x | |
| AQT | x | x | x | x | |
| TMTA | x | x | x | x | |
| TMTB (if MMSE ≥20) | x | x | x | x | |
| ADAS 10 word recall + recognition + BNT15 | x | x | x | x | |
| SDMT | x | x | x | x | |
| Letter S fluency | x | x | x | x | |
| Animal fluency | x | x | x | x | |
| VOSP incomplete letters | x | x | x | x | |
| VOSP cube analysis | x | x | x | x | |
| HADS | x | x | x | x | |
| Motor aspects and visuospatial navigation |
† Reduced scheme for a subgroup of amyloid negative participants (see PET table below).
Table Cohort D & E
Follow-up of cases with dementia (Cohorts D and E) AD, VaD, and FTD
| Baseline | 6 m | 1 y | 2 y | |
| MD/Nurse | MD/Nurse | MD/Nurse | MD/Nurse | |
| MRI | x | x | ||
| Tau PET | x | x | ||
| Amyloid PET | x** | x** | ||
| Lumbar puncture | x | x | ||
| Plasma/Blood | x | x | ||
| Basic Demographics | x | |||
| BAS+ and Sleep questionnaires | x | x | ||
| CIMP-QUEST | x | x | x | |
| ADL | x | x | x | |
| EQ-5D (VAS also informant) | x | x | x | |
| MBI-C | x | p.r.n. | p.r.n. | x |
| MMSE | x | x* | x | x |
| AQT | x | x* | x | x |
| TMTA | x | x* | x | x |
| TMTB (if MMSE ≥20) | x | |||
| ADAS 10 word recall + recognition + BNT15 | x | x | x | |
| SDMT | x | |||
| Letter S fluency | x | |||
| Animal fluency | x | x | x | |
| VOSP incomplete letters | x | x | x | |
| VOSP cube analysis | x | |||
| Cognitive tests besides MMSE are only done at follow-up if MMSE >13 p | ||||
* Only if the patient was included from Memory Clinic, Skåne University Hospital. ** Only in a subgroup of 100 participants. p.r.n.: pro re nata, when needed.
- From 2018-NOV-13 MBI-C, EQ-5D and CIMP-QUEST will not be performed at the 1-year follow-up
Table Cohort E parkinsonian
Follow-up of cases with dementia (Cohorts E)
Parkinsonian disorders†
| Baseline | 6 m | 1 y | 2 y | |
| MD/Nurse | MD/Nurse | MD/Nurse | MD/Nurse | |
| MRI | x | x | ||
| Tau PET | x | x | ||
| Amyloid PET | ||||
| FDOPA PET | x | x | ||
| Lumbar puncture | x | x | ||
| Plasma/Blood | x | x | ||
| Skin Biopsy | x | x | ||
| Basic Demographics | x | |||
| BAS+ and Sleep questionnaires | x | x | ||
| CIMP-QUEST | x | x | x | |
| ADL | x | x | x | |
| EQ-5D (VAS also informant) | x | x | x | |
| MBI-C | x | p.r.n. | p.r.n. | x |
| SCOPA-AUT | x | x | x | |
| RBD screening questionnaire (RBDSQ) | x | x | x | |
| SCOPA-sleep | x | x | x | |
| HADS | x | x | x | |
| NMSQuest | x | x | x | |
| MMSE | x | x* | x | x |
| AQT | x | x* | x | x |
| TMTA | x | x* | x | x |
| TMTB (if MMSE ≥20) | x | |||
| ADAS 10 word recall + recognition + BNT15 | x | x | x | |
| SDMT | x | |||
| Letter S fluency | x | |||
| Animal fluency | x | x | x | |
| VOSP incomplete letters | x | x | x | |
| VOSP cube analysis | x | |||
| Sniffin’ Sticks test | x | x | ||
| Motor aspects and visuospatial navigation | x | x | ||
| Cognitive tests besides MMSE are only done at follow-up if MMSE > 13 p | ||||
* Only if the patient was included from Memory Clinic, Skåne University Hospital. † parts of the protocol also includes participants in other cohorts if suspicion of parkinsonian disorder during follow-up, which is performed at two occasions with three years in between. p.r.n.: pro re nata, when needed.
- From 2018-NOV-13 MBI-C, EQ-5D and CIMP-QUEST will not be performed at the 1-year follow-up.
- FDOPA PET, SCOPA-AUT, RBDSQ, SCOPA-SLEEP, NMSQuest and Sniffin’ Sticks test were incorporated from 2019-JUN-10
Table PET follow-up
Amyloid PET
Tau and Amyloid PET Follow-up
| Baseline | 2 y | 4 y | 6 y | 8 y | |
| Cohort A & B – Full FU | x | x | x | x | x |
| Cohort A & B – Reduced FU | x | x | x | * | |
| Cohort A2 – Full FU | x | x | x | ||
| Cohort A2 – Reduced FU | x | x | x | ||
| Cohort C1 & C2 – Full FU | x | x | x | x | |
| Cohort C1 & C2 – Reduced FU | x | x | x | ||
| Tau PET | |||||
| Baseline | 2 y | 4 y | 6 y | 8 y | |
| Cohort A & B – Full FU | x | x | x | x | x |
| Cohort A & B – Reduced FU | x | x | x | * | |
| Cohort A2 – Full FU | x | x | x | ||
| Cohort A2 – Reduced FU | x | x | x | ||
| Cohort C1 & C2 – Full FU | x | x | x | x | x |
| Cohort C1 & C2 – Reduced FU | x | x | x | ||
| Cohort D & E – Full FU (All participants) | x | x |
Approximately 2/3 of amyloid negative participants in cohort A, B, C perform a reduced PET follow-up scheme, named “Reduced FU” above. Cohort A2 differentiate from the other controls in their full follow-up, thereof that these are specified separately. Other investigations (clinical, cognitive test, MRI, LP) are performed according to standard study protocol. If the study is prolonged for healthy control the 8y PET for the participants with reduced FU will be performed at 10 y.