Overview
Idiopathic RBD
ENROLLMENT
The study recruits 100 individuals with idiopathic REM-sleep Behavioural Disorder (iRBD). Individuals with iRBD are recruited from: A) Memory Clinics, Neurology clinics and Neurofysiology clinics at Skåne University Hospital, Helsingborg Hospital, and Ängelholm Hospital in southern Sweden; B) specialized Sleep Clinics in Sweden; C) through advertisements. iRBD is defined according to the AASM criteria and requires verification by polysomnography.
INCLUSION CRITERIA
- Polysomnography-verified RBD according to AASM criteria.
- Does not fulfill diagnostic criteria for idiopathic Parkinson´s disease.
- Age range 50-100. Women who are <55 years of age will be required to take a pregnancy test before participation in the PET, MRI and scintigraphy part of the study if not post-menopausal.
- Ability to give informed consent.
- Speaks and understands Swedish to the extent that an interpreter is not necessary for the patient to fully understand the study information and cognitive tests.
EXCLUSION CRITERIA
- Past history of severe or repeated concussive head injury or stroke or any significant systemic disease or unstable medical condition.
- History of severe and unstable depression, schizophrenia, schizoaffective disorder or bipolar disorder.
- Significant white matter microvascular disease.
- Contraindication to MRI and PET.
FOLLOW-UP FOR 8 YEARS
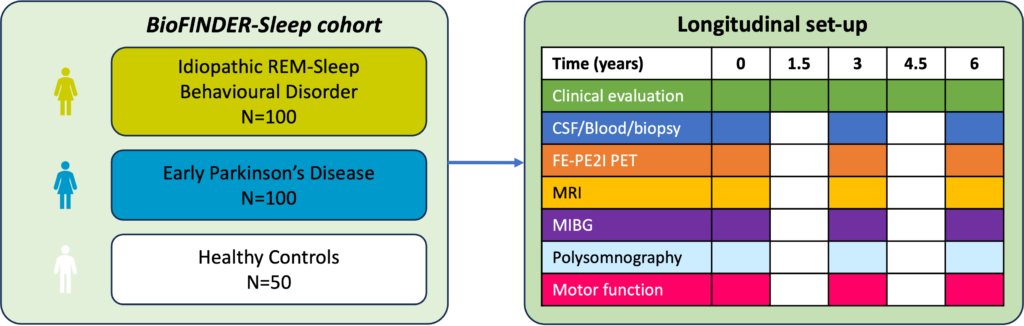
Clinical assessments and evaluations are repeated after 1.5 years, 3 years, 4.5 years and 6 years. CSF/Blood sampling, MRI, FE-PE2I PET, MBIG and polysomnography are performed after 3 and 6 years as described in table below.
Early Parkinson’s Disease
ENROLLMENT
The study recruits 100 patients with early Parkinson’s Disease (PD). Early PD patients are recruited from Memory Clinics and Neurology clinics at Skåne University Hospital, Helsingborg Hospital, and Ängelholm Hospital in southern Sweden. Early PD is defined as de novo (without any PD treatment) of with treatment for maximum of three years.
INCLUSION CRITERIA
- Fulfills the diagnostic criteria for idiopathic Parkinson´s disease.
- The PD patients will be de novo (yet without any PD treatment) or with treatment for maximum of 3 years.
- Age range 50-100. Women who are <55 years of age will be required to take a pregnancy test before participation in the PET, MRI and scintigraphy part of the study if not post-menopausal.
- Ability to give informed consent.
- Speaks and understands Swedish to the extent that an interpreter is not necessary for the patient to fully understand the study information and cognitive tests.
EXCLUSION CRITERIA
- Past history of severe or repeated concussive head injury or stroke or any significant systemic disease or unstable medical condition.
- History of severe and unstable depression, schizophrenia, schizoaffective disorder or bipolar disorder.
- Significant white matter microvascular disease.
- Contraindication to MRI and PET.
- Normal dopamine transporter ([18F]FE-PE2I) scan.
FOLLOW-UP FOR 8 YEARS
Clinical assessments and evaluations are repeated after 1.5 years, 3 years, 4.5 years and 6 years. CSF/Blood sampling, MRI, FE-PE2I PET, MBIG and polysomnography are performed after 3 and 6 years as described in table below.
Healthy Controls
ENROLLMENT
The study recruits 50 healthy controls. They are mainly recruited through advertisements, but other means are also used, e.g., relatives to participating patients if not related by blood.
INCLUSION CRITERIA
- Age range 50-100. Women who are <55 years of age will be required to take a pregnancy test before participation in the PET, MRI and scintigraphy part of the study if not post-menopausal.
- No diagnosis of PD or another significant neurological disorder.
- No diagnosis of RBD.
- Ability to give informed consent.
- Speaks and understands Swedish to the extent that an interpreter is not necessary for the patient to fully understand the study information and cognitive tests.
EXCLUSION CRITERIA
- Past history of severe or repeated concussive head injury or stroke or any significant systemic disease or unstable medical condition.
- History of severe and unstable depression, schizophrenia, schizoaffective disorder or bipolar disorder.
- Significant white matter microvascular disease.
- Contraindication to MRI and PET.
FOLLOW-UP FOR 6 YEARS
Clinical assessments and evaluations are repeated after 1.5 years, 3 years, 4.5 years and 6 years. CSF/Blood sampling, MRI, FE-PE2I PET, MBIG and polysomnography are performed after 3 and 6 years as described in table below.
Table Study Procedures
| Early PD & iRBD patients | |||||
| Baseline | 1.5 y | 3 y | 4.5 y | 6 y | |
| Cognitive testing | |||||
| MMSE | x | x | x | x | x |
| AQT | x | x | x | x | x |
| TMTA | x | x | x | x | x |
| TMTB | x | x | x | x | x |
| ADAS 10 word recall + recognition + BNT15 | x | x | x | x | x |
| SDMT | x | x | x | x | x |
| Letter S fluency | x | x | x | x | x |
| Animal fluency | x | x | x | x | x |
| VOSP incomplete letters | x | x | x | x | x |
| VOSP cube analysis | x | x | x | x | x |
| Questionnaires | |||||
| Basaldataformulär/allmänna patientuppgifter | x | ||||
| BAS questionnaire | x | x | x | x | x |
| CIMP-QUEST | x | x | x | x | x |
| ADL | x | x | x | x | x |
| EQ-5D | x | x | x | x | x |
| Non-Motor Symptom Scale (NMSS) | x | x | x | x | x |
| SCOPA-AUT | x | x | x | x | x |
| Bristol stool scale & ROME-III | x | x | x | x | x |
| RBD screening questionnaire (RBDSQ) | x | x | x | x | x |
| Epworth Sleepiness Scale (ESS) | x | x | x | x | x |
| Questionnaire for Impulsive-Compulsive Disorders in Parkinson’s Disease (QUIP) | x | x | x | x | x |
| Food Frequency Questionnaire (FFQ) | x | x | x | x | x |
| HADS | x | x | x | x | x |
| Motor tests | |||||
| MDS-UPDRS | x | x | x | x | x |
| Hoehn & Yahr | x | x | x | x | x |
| Tandem gait test | x | x | x | x | x |
| Arm-hand test | x | x | x | x | x |
| Purdue-pegboard | x | x | x | x | x |
| Detailed motor assessments and dual tasking, e.g. using an electronic walkway | x | x | x | ||
| Imaging | |||||
| MRI 3T | x | x | x | ||
| MRI 7T (subpopulation) | x | ||||
| [123I]MIBG SPECT | x | X | x | ||
| [18F]FE-PE2I | x | x | x | ||
| [123I]ioflupane SPECT | (x) | ||||
| Other procedures | |||||
| Polysomnography | x | x | x | ||
| Plasma/Blood | x | x | x | ||
| Lumbar puncture | x | x | x | ||
| Skin biopsy* | x | x | x | ||
| Sniffin’ Sticks test (’Screening 16 test’ Burghart, Wedel, Germany) | x | x | x | x | x |
| Smarthphone-based assessment of motor and cogntive functions | x | x | x | x | x |
*Repeated skin biopsy collection can be done on up to five occasions